One day, a boy (let's call him "Jake") decided to cook a lovely breakfast for his special lady friend (let's call her "Katie"). He planned out the perfect meal, and he knew Katie would love it. (They had been dating almost ten years, after all, and Jake knew what foods Katie liked.) He set about to frying a mess of sliced potatoes, a thick slab of maple-flavored bacon, and a panful of the most glorious scrambled eggs ever invented with tomatoes, peppers, green beans and extra-sharp cheddar cheese. Oh, the cheese! The kitchen was filled with a harmonious collaboration of breakfast smells, clanking pots, and banjo music streaming from a laptop on the kitchen table.
Now, being as it was early morning, the potential for wildlife-based distractions was high. And sure enough, Jake looked casually out the window toward his lush garden oasis. What did he see? A ne'er-do-well cottontail rabbit working his way through the rows of green beans that had just begun to ripen. Didn't that varmint know better than to work in the beans while there was still dew on them?! This situation simply could not be allowed to unfold without intervention. Jake grabbed his trusty home-made blow gun and snuck out to the garden, intent on adding some rabbit to the breakfast menu.
Despite his best efforts, Jake got to the garden just in time to see the rabbit disappear through a hole in the fence at the back corner. "So that's how he'd gotten in! Well, I better fix up this fence right away or he'll be in there again by the time I get back to the house," thought Jake to himself. He went to the garage to get the pliers and wire cutters, and a shovel to re-bury the bottom part of the fence. Fifteen minutes later, there's no way that rabbit's getting back in here! Hey, what's that smell?
Uh-oh.
The potatoes, eggs, and bacon were barely recognizable. The harmonious scents of breakfast had turned to an acrid cloud billowing from the stove; the banjo music from the laptop was now part of a cacophony that also included a roommate's alarm clock (forgotten to turn off before leaving for the weekend) and every smoke alarm in the house. So much for surprising Katie with breakfast in bed! She's probably standing outside in the designated meeting area waiting for the firetruck to show up.
If you've been cooking for more than a few months, you might have a similar story about burning food (maybe without the banjo music and blow guns). After finally convincing the smoke alarms to shut up and airing out the house, the typical follow up question is, "How am I going to get this mess out of these pans?" A quick web search reveals that people have probably been trying to answer this question since the beginning of time.
It seems that there are five commonly attempted routes to get food residues off pots and pans:
Add acid
Vinegar (acetic acid)
Cream of Tartar
Citric acid
Barkeeper's Friend (oxalic acid)
Soda (carbonic acid)
Add base
Baking soda (sodium bicarbonate)
Oven or drain cleaner (sodium hydroxide, or lye)
Ammonia cleaner
Add solvent (can't recommend the last one)
Alcohol (as in deglazing a pan with wine)
Detergent such as soap or dishwashing liquid
Nail polish remover (acetone)
Gun-cleaning solvent (hydrocarbon cocktail)
Add oxidant (also can't recommend the last two)
High heat (above 900 °F or 500 °C)
Bright sunlight
Bleach (sodium hypochlorite)
Oxyclean
Add abrasive (and scrub)
Scouring powder
Salt
Eggshells
Another one that shows up occasionally is rapid heating and cooling of the soiled pan, which is likely an attempt to take advantage of the difference in coefficients of thermal expansion between the metal pot and the carbonaceous film coating it. Since the difference is probably pretty small, and most films are somewhat malleable anyway, this technique is probably not going to be more efficient than the above mentioned ones.
Explanations of why each of these methods work chemically are few and far between, but there are plenty of anecdotal descriptions of each not working in some cases and working like a charm in others. The variety of experiences probably comes from the varying combinations of food types and pans, conditions of forming the residue (temperature, aerobic/anaerobic conditions, cook time), and patience level of the anecdote suppliers. In truth, the exact mechanism of each route probably hasn't been conclusively determined, but we can get some insight by considering what types of chemistry are likely going on.
When food 'burns' on a stove, it normally starts (in our experience) as a few spots on the bottom of the pan that darken. What reactions produce this darkening depends on the type of food in the pan. The food is undergoing a complex process called pyrolysis, which happens in limited oxygen and consists of food molecules simultaneously breaking down into smaller compounds and building up (polymerizing) into larger compounds. The temperature and oxygen availability help to determine what the ratio of 'breaking down' to 'building up' is. Higher temperatures and more oxygen lead to more 'breaking down.'
For the potatoes in the story above, which are mainly composed of starch (a carbohydrate polymer), the reactions are mainly hydrolysis and dehydration reactions, converting the carbohydrate polymers first into simple sugars like glucose (or glucose anhydrides), then dehydration products (oxygen-functionalized aromatic molecules (e.g., phenol) and re-polymerized sugars), and eventually, carbon. For the eggs and bacon, which contain a lot of proteins, an analogous depolymerization and further reaction of the amino acid chains occurs, but incorporates many nitrogen-containing functional groups in the aromatic molecules (e.g. benzonitrile) as well. The bacon grease, which is made up of triglycerides, breaks down first into glycerol and fatty acids; the glycerol dehydrates like a carbohydrate, and the fatty acids can polymerize or break down into smaller hydrocarbons. The proteins and carbohydrates can also react together through the Maillard reaction, which makes that nice golden brown color, but also less healthy compounds. There are also minerals like potassium and calcium carbonates and hydroxides involved, but these components are probably not the main concern in your carbonaceous mess.
 |
| Example of how milk protein can go from a nutritious component of a white sauce to a useless black glob stuck to the bottom of your pan. Fortunately, you can remove it with an alkaline solution (pH > 7, but easier with 9 < pH < 12), e.g. with baking or washing soda. Image credit: Wikipedia. |
The general trend is that the higher the heat and the longer the cook time, the closer to pure carbon you get. Concomitantly, the closer to carbon you get, the less effective each cleaning technique becomes. In fact, pure carbon is typically insoluble in acids, bases, and most common solvents, so the only real options if you get to that point are oxidation and abrasive cleaning. In each case, the problem to solve (ba-da-bum!) is the chemical bonds formed between the food and the metal pan (e.g., iron or aluminum). You have to convince the metal and the food that they should not like each other. The trick is that the different types of food respond differently to the different treatments. For example, the Arizona Department of Health Services discusses that protein residues respond well to addition of basic components, while adding an acid can actually make the problem worse. They offer this guide to clarify what will typically work to clean your pans based on what type of food you were trying to cook.
Why do basic solutions work well for multiple types of food? The key lies in the versatility of the hydroxide ion, OH-. When bases dissolve in water, they release a hydroxide ion into solution, which can react with many types of organic molecules. With fats and greases, the hydroxide ion is able to catalyze the saponification reaction, which essentially turns the grease into soap. (...and which is also why lutefisk can tend to taste soapy). So in this case, you get both a detergent and a dissolution effect from the base. The carbohydrate-based residues can be removed by the saponified grease. The hydroxide ion also interferes with the chemical bonding in protein residues (especially of sulfur-sulfur linkages), which denatures the proteins into water-soluble pieces. Triple whammy!
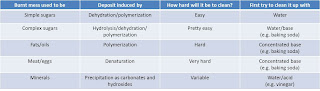 |
| Table with recommendations on how to clean up food messes depending on their origin. Adapted from Arizona DHS. |
The acids typically work best on mineral deposits, but can also act as a solvent (think of the acetic acid in vinegar as kind of a hybrid between water and acetone). Both acids and bases may also serve to corrode a thin layer of the metal pan, which can release the food residues. Also for both acids and bases, the rate at which they work their magic goes up with temperature. So if you heat them in boiling water, they'll work faster. Stronger acids and bases work faster than weak ones, so lye will work faster than baking soda, for example. (Ok, it should formally depend on the concentration of each, but practically speaking, lye will work faster).
Oxidation converts the deposits mainly to CO and CO2, using either an aqueous oxidant, such as in bleach or Oxyclean, or using O2 with either a photochemically-induced (bright sunlight) or thermally-induced (high temperatures) reaction. The mechanism of abrasion is pretty self explanatory--you use something harder than the deposit to grind it off. Of course, be careful with abrasives on nonstick surfaces.
 |
| General trends for making burnt food messes and how to clean them up. |
Essentially, what it comes down to is that for most food residues, your most practical (read cheap, safe, and easy) approach will probably be to make a concentrated solution of baking soda in water in the pan with the residue, bring it to a boil, and let it sit overnight. In the morning, try to scrub the residue off. If it doesn't work, add some kind of abrasive (pending the hardness of your cooking surface) and scrub some more. If you still can't get it, go the oxidation route.
Do you have any other techniques for cleaning burned food residue off pots and pans? Tell us about it in the comments section below!

When I occasionally have tough food to remove from a pan (doesn't always have to be burned on, just cooked on), I find first adding water to the hot pan (deglazing it) will often help. Then if that is not enough, if the pot is able to be placed onto a burner (metal cake pans can be placed across 2 burners), I boil the pot with some water up to the food line...then throw in some baking soda. It will fizz up and look very impressive. So I repeat it a few more times. Then let it boil a bit longer, turn off the burner, and let it sit overnight. It is usually amazingly easy to remove the food residue, give it a good wash with some soap, and it's done!
ReplyDeleteck
Thanks Mom! It's good to have an actual procedure to follow--I should have included that in the post. Good thing you're on top of it! :-)
DeleteHi, I found this site after googling how to take care of burnt tomato sauce in a enameled/cast iron pot. I put baking soda, then the boiling water, left it overnight. It did soften up the food enough that a little scraping was all that was needed!
DeleteBoy does this post make me glad my husband does all the dishes. :-)
ReplyDeleteAhh, but now you know some of the secrets to getting them clean easily! Thanks for the comment!
DeleteI am NOT shilling this but, years ago in college, a friend decided to go to the Amway route and demoed some kind of special solvent they had foe carbonized pans. It was truly amazing, but I have no idea if it was a prepared set up.
ReplyDeleteFound your interesting article here trying to figure out how to help a friend and thought there might be some commercial equivalent product of the hardware store. I guess I'll try the baking soda route first.
Friends problem must be related to milk-dairy. She was trying to make sour cream!
Ok, Jake. This was an amazing experience. I bought the Arm & Hammer washing soda and, per its instructions, created a paste on top of the carbon and let it sit on the counter overnight (rather than the recommended 30 minutes). Horrors! In the morning it was a second layer of hardened white soda crystals. At this point I put it on the stove on high heat and the crystals reliquefied. I let it boil for a few minutes and then began to work the carbon on the bottom with a standard steel spatula. ( A firm putty knife would have worked just as well). Eventually all the carbon came up and mixed with the liquefied soda crystals.
DeleteDor the final cleaning, I took the Bar Keepers Friend compound and and a dry erase type of sponge and I can now see my face in the bottom of the pan. Thanks so much.
(most interesting part of the literal "kitchen chemistry" was observing the "skin" which developed on the surface of the water as the soda and carbon reacted with each other).
Hi Neill, thanks for stopping by and sharing your experience! I'm glad you were able to get the pan clean with the washing soda and scouring powder. Good to know that after the main carbon layer is off, I can polish it up with BKF. Now the question is, was it the oxalic acid or the abrasive in the BKF that did the job? I smell another experiment...
DeleteThis comment has been removed by the author.
DeleteHi Neill, that does sound like an excellent application of whatchagotamology!
DeleteI'm not sure if Blogger's platform allows pictures within a comment, but if you can upload them to your Google+ profile or somewhere like Flickr or Instagram, you can post links to the photos using the syntax link text. Looking forward to seeing this bike tire clamp!
Sorry, that didn't post right. This page gives the syntax to use.
DeleteSo, Jake -
DeleteI just had a wonderful result repairing an old bureau (with mirror) where the laminate exterior had gone bad, i.e., was coming up off the underlying wood on the surface of the main drawer.
Again, not shilling for a particular product, but I used Gorilla brand wood glue. (I'm sure that Elmers would work just as well here. Or perhaps readers of this site already know how to make their own? Horses hooves? Not going there…..)
Anyway, two possible points of interest to your blog-readership.
How to apply a clamp - and what kind to a large drawer. Didn’t want to take it apart…
Working with glue is messy, and it extrudes from the seams once the clamp is applied.
The solutions:
as a clamp item I used old bicycle tires freely given by the owner of the local shop. They wrap beautifully around any unusual configuration and can be adjusted to any lengths and tension all the way around of the project, in this case case the drawer.
I wanted to create a barrier-layer between the glue (which would extrude) and the tubes. A magazine cover or ordinary cardboard would be adhered to by the glue, so I tried the cellophane wrapper from a box of Cheerios.
Voila! The results could not be better - see pictures below. (Sorry, wish I had one of the bad spot before I pushed in the glue.)
https://plus.google.com/115946267689572339268/posts/TUU6map1MwV
So it it is your job to get this over to Whatchgotamology.....
DeleteJake, my only regret is that I didn't take a "before and after" picture of the process for posting here.
ReplyDeleteHow do I put on the label for Whatchgotcah so it posts over there? This one isn't about Food!
ReplyDeleteI don't think I can tag comments, so I'll have to turn it into a post and add the tag there. It'll probably take me a few days, but we'll get it there eventually!
DeleteHave you heard of the trick using water, soap and a dryer sheet? I imagine it has to do with the positive static charge on the sheet but I'd love it to be analyzed properly
ReplyDeleteSame here!
Delete