We've been stewing over something for a long time, and it came to the surface again this last week while building our
picnic table benches. So, we're going to do something we don't normally do in public: passionately rant about mundane objects. After all, it's time someone just got it out there: Phillips-head wood screws are stupid. They're not as stupid as the flat-head screws they were designed to replace, but they are not much better.
 |
| Sometimes, preschoolers have the most appropriate terminology. |
What's the problem, you ask? Let's play a game. Raise your hand if you've ever tried driving a Phillips-head screw longer than about three inches into a piece of wood without a pilot hole. Keep it raised if you were able to drive that screw all the way in without the bit slipping out of the head. Any hands still up? Ok, keep it raised if you were able to do ten in a row without destroying any screws or any driver bits. No hands up yet? Good! We knew we weren't alone. And thank you for being honest.
So, why are they so hard to drive consistently? There are at least three reasons. First, consider that the job is a lot easier if you drill a pilot hole because the screw itself has to push a lot less wood out of the way (i.e., a lot less torque is required to keep the screw moving into the wood). Now, consider that the diameter of the screw shank is normally pretty thin, and the drill bits that match are normally pretty short. The correct-diameter bits in our set, for example, will only make a hole about 2" deep. That is to say, a four-inch long Phillips-head screw with a 1/8" shank diameter has no place in a civilized society. Philips screws shorter than 2.5" are slightly more functional, but if you're building a deck or a picnic table (and not doing fine woodworking), do you really want to drill pilot holes for every dang screw? Ain't nobody got time for that.
 |
| Here, let me drill a woefully inadequate pilot hole for you. |
The second reason is that electrically-powered
screwdrivers (e.g., drills) are very common these days, so if you don't have sufficient
force or just the right angle, you can strip out the head or destroy your
driver bit (depending on which is made from harder metal) really
fast. You can do the same thing with a hand-powered screwdriver, but it
takes a lot longer. (Side note: we can't believe
this is a real thing. Possibly the least-elegant solution ever offered by a real company. That alone suggests Phillips heads should be allowed to go extinct.)
The third, most nefarious reason is that, depending on who you ask, Phillips-head drivers are either
designed to slip out of the screw head (ostensibly to avoid overtightening), or that just happens to be a feature that someone along the line decided was marketable. Either way, Phillips
put it in a patent, so they have to own it now. That means it's not an accident that your Phillips driver bit has only four tiny triangles through which to apply force to the screw. (Some may say that a limited amount of sympathy is due for the relatively primitive screw manufacturing technology of the time, but the Robertson square bit
was patented earlier and is a much better design.) In any case, an inherent driver-disengage feature might make sense in fragile applications, but not for regular homestead-style outdoor construction. According to
this fascinating and surprisingly-well-referenced Wikipedia article, Philips has come up with some variations to try to improve matters, but they can't get around the fact that they're tied to a design that sucks.
 |
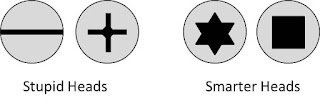
| We've always been bigger fans of quadrilaterals than triangles, even before we studied physics. |
Now, we're not hardware experts around here, but we have done quite a bit of...um...we've driven quite a few screws in our day, and we plan to drive many more. The Torx (star) screws have become common enough that the prices are comparable to Phillips screws, especially when factoring in all the destroyed Phillips screws and driver bits, extra time, and tears of frustration. So, we've taken a vote, Mr. Phillips, and you are off the island.
That is all.
 |
| Here's a tip as a reward for reading this rant all the way to the end: the Torx screws are so much better that if you've already got a supply of really long Phillips screws, you can use a Torx screw of similar length to make a decent pilot hole for the Philips screw. That way you don't have to throw anything out or make an unethical business choice to sell the Phillips screws at a garage sale. |